Benefits of Digital SOPs for Laboratory Compliance and Efficiency
Standard operating procedures (SOPs) are critical in the pharmaceutical business for ensuring laboratory compliance and efficiency. However, managing laboratory protocols and data can be difficult, particularly when paper-based SOPs are used. Digital SOPs solve these issues by enabling pharmaceutical firms to streamline laboratory documentation and promote compliance. In this blog post, we’ll look at the Benefits of Digital SOPs and how they can help laboratories increase efficiency and compliance.
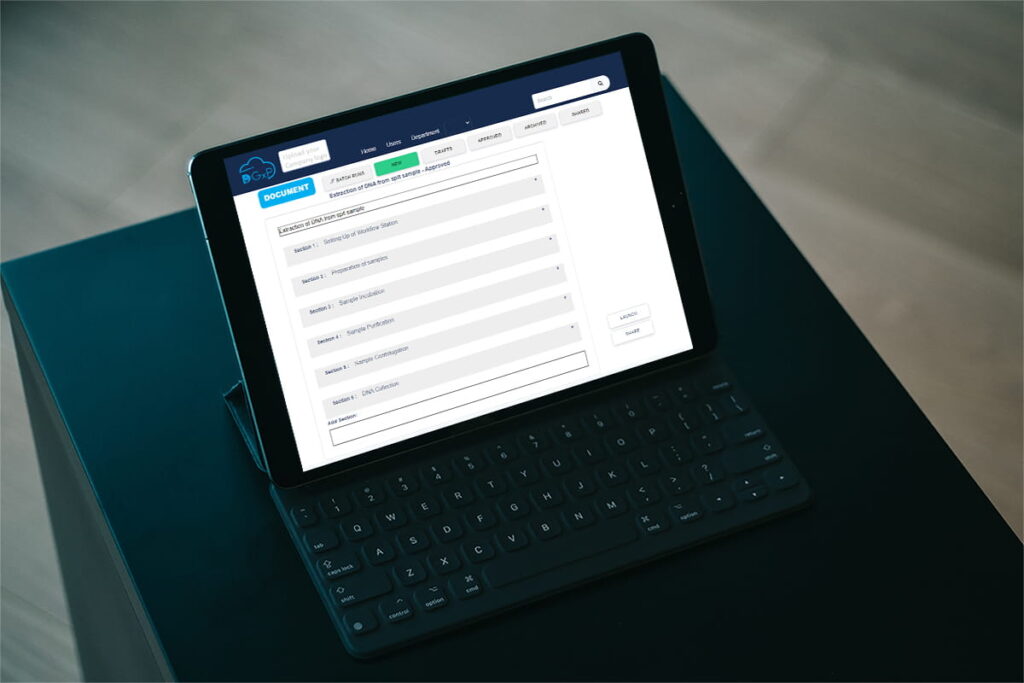
What are Digital SOPs?
Digital SOPs, or digital standard operating procedures, are electronic documents that outline the steps required to complete a specific task in the laboratory. These procedures are created and stored electronically, and can be accessed by laboratory personnel from a digital device, eliminating the need for paper-based SOPs. Video SOPs, which offer step-by-step video instructions, are a popular form of digital SOPs.
Benefits of Digital SOPs for Laboratory Compliance
Digital standard operating procedures (SOPs) have become an essential tool for ensuring laboratory compliance in the pharmaceutical industry. Here are some of the specific benefits that Digital SOPs offer for laboratory compliance:
Improved consistency
Improved consistency is a specific benefit of digital SOPs for laboratory compliance. Digital SOPs ensure that all laboratory personnel follow the same procedures, lowering the risk of human error and data inconsistency. Laboratory personnel can be trained to follow standardized procedures using Digital SOPs, ensuring that data is collected and analyzed consistently across all labs. This consistency is important for regulatory compliance because it ensures that all laboratory activities are carried out consistently.
Enhanced Traceability
Traceability is another benefit of digital SOPs. Digital SOPs can be tracked and audited, providing a comprehensive record of laboratory activities and ensuring regulatory compliance. This traceability is critical for maintaining regulatory compliance, as it provides a clear audit trail of laboratory activities. With Digital SOPs, laboratory personnel can easily track and document their work, providing an accurate and complete record of laboratory activities.
Active Version control
Digital SOPs are simple to update and distribute to laboratory personnel, ensuring that everyone is using the most recent version. When using paper-based SOPs, it can be difficult to keep track of which version is the most recent, which can lead to confusion and errors. Digital SOPs solve this issue by providing laboratory personnel with access to the most recent version of the SOP, ensuring that everyone is working from the same set of instructions.
Secured Access control
Digital SOPs can be restricted to authorized personnel, ensuring that sensitive laboratory protocols are only accessed by trained and qualified personnel. This access control is essential for the security of laboratory protocols and data. Laboratory managers can protect sensitive information by restricting access to authorized personnel, reducing the risk of data breaches or other security incidents.
Improved Laboratory Safety Practices
Digital SOPs can help ensure that laboratory safety practices are consistently followed, lowering the risk of accidents and ensuring that laboratory staff adhere to best practices. By using digital SOPs, you can more effectively communicate and demonstrate safety protocols, making it easier for your employees to understand and follow them.
Furthermore, digital SOPs can facilitate more efficient safety documentation and reporting. This can lead to improved compliance and, ultimately, a safer laboratory environment for all employees.
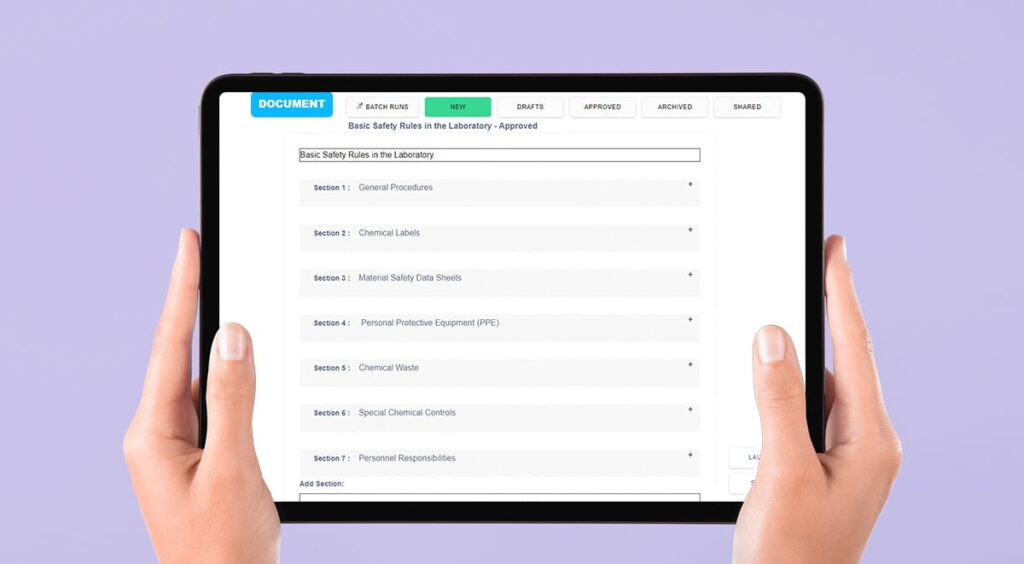
Benefits of Digital SOPs for Laboratory Efficiency
Digital SOPs not only improve laboratory compliance but also boost laboratory efficiency. Below are some of the ways Digital SOPs can make laboratory operations more efficient:
Increased Time savings
Digital SOPs eliminate the need for paper-based documentation, reducing data entry and paperwork time. With Digital SOPs, laboratory personnel can electronically complete forms and documentation, streamlining data entry and reducing documentation time. This time savings is especially important in a laboratory setting where time is a valuable commodity.
Efficient Data management
Integrating digital SOPs with laboratory information management systems (LIMS) and electronic laboratory notebooks (ELN) allows for seamless data management and analysis. Laboratory personnel can easily record and analyze data through combining Digital SOPs with LIMS and ELN, improving laboratory efficiency and lowering the risk of errors. This integration can also make it simpler to track and monitor laboratory activities, increasing laboratory efficiency.
Ensured Laboratory best practices
Digital SOPs can be used in the laboratory to promote best practices by ensuring that laboratory personnel follow standardized procedures, promoting efficient and consistent laboratory practices. Laboratory managers can use Digital SOPs to provide standard processes that encourage the use of best practices to their employees. Therefore, Digital SOPs can help to eliminate wasteful practices, reduce errors, and improve laboratory efficiency by standardizing laboratory best practices.
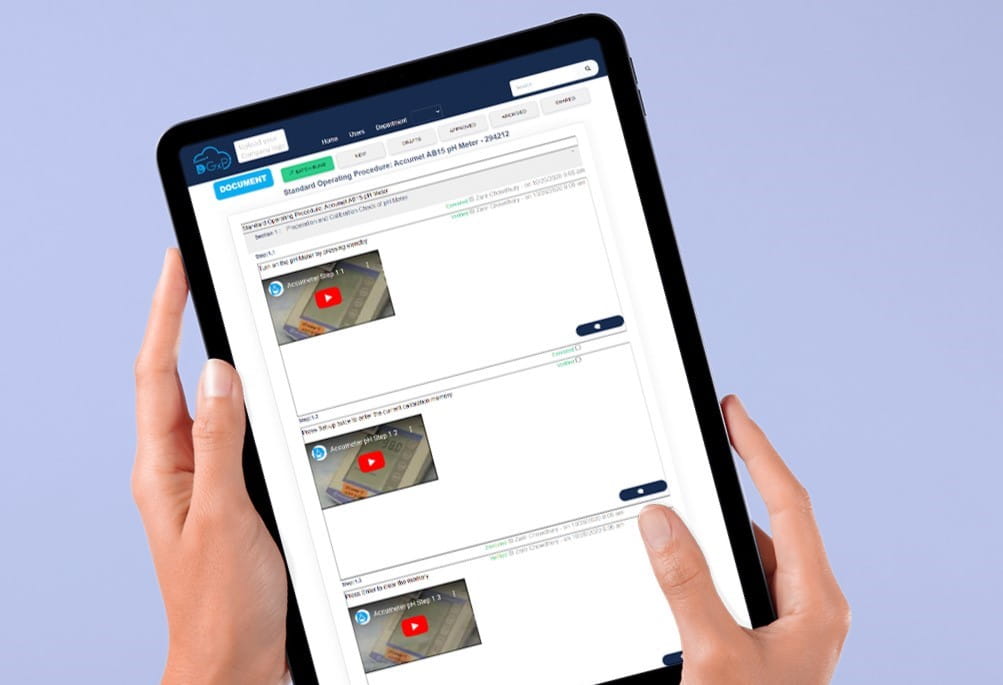
Other features that can enhance Digital SOPs
Video SOPs for Enhanced Learning and Compliance
Video SOP is an effective tool for improving learning and compliance in your laboratory. It can enable you to demonstrate procedures visually and audibly, making it easier for your employees to understand and follow them. You can use video SOPs to ensure that your employees perform tasks correctly and consistently, decreasing errors and increasing compliance.
Video SOPs can also help with the onboarding of new employees. You can help them learn faster and more effectively by showing them visual and auditory demonstrations of procedures. This saves time and money on training while also ensuring that procedures are followed correctly from the start.
Electronic Batch Records for Real-time Data Monitoring
To provide a comprehensive view of laboratory processes, digital SOPs can be integrated with Electronic Batch Records (EBRs). You can ensure real-time monitoring of laboratory data management by integrating digital SOPs with EBRs, ensuring that laboratory methods and techniques are consistently followed. This can result in a more accurate and streamlined approach to data management, as well as a reduction in the risk of errors and inconsistencies.
Furthermore, this integration may enable more efficient record-keeping and documentation of laboratory processes, making data tracking and analysis easier. This can lead to increased compliance and, ultimately, better decision-making.
Laboratory Information Management System for Streamlined Workflow
To provide a complete laboratory workflow optimization solution, digital SOPs can be integrated with a Laboratory Information Management System (LIMS). This integration can aid in the automation of laboratory data management and the elimination of manual data entry errors. LIMS can manage massive amounts of data, and digital SOPs can ensure that this data is accurate and consistent, lowering the possibility of errors.
Integrating digital SOPs with LIMS can provide a more streamlined approach to laboratory workflows, reducing the time and resources required to complete specific tasks. This can lead to increased productivity and, ultimately, higher work quality.

In conclusion, digital SOPs offer several benefits for laboratory compliance and efficiency in the pharmaceutical industry. By adopting digital SOPs, pharmaceutical companies can streamline their laboratory protocols and data management, promote good documentation practices, and ensure compliance with regulations.
If you’re looking for a digital documentation software solution for your pharmaceutical laboratory, consider DigitalGxP. Our software offers features such as digital SOPs, video SOPs, electronic batch records, electronic laboratory notebooks, and laboratory information management systems.
It provides a comprehensive solution for all your documentation needs and can help ensure regulatory compliance, reduce errors, increase productivity, and improve overall lab performance. It is definitely worth considering for any pharmaceutical laboratory looking to streamline their processes and achieve greater success.
So, sign up today to find out how we can assist your laboratory in achieving compliance and efficiency.