Why Electronic Lab Notebooks Are Essential for R&D in Pharma
Pharmaceutical research and development involve complex laboratory procedures, which yield extensive data. It is crucial to capture, manage, and share laboratory data efficiently and accurately to ensure regulatory compliance, reduce documentation errors, and accelerate the drug development process. However, traditional paper-based laboratory notebooks and documentation systems can no longer cope with the demands of modern research and development.
This is where electronic lab notebooks (ELNs) come into play, offering a digital solution for laboratory data management. In this post, we’ll delve into the reasons why Electronic Lab Notebooks are indispensable for research and development in the pharmaceutical industry.
What are Electronic Lab Notebooks?
Electronic lab notebooks (ELNs) are essentially digital versions of traditional paper notebooks. Scientists and researchers use them to record experiments, observations, and findings. These software applications provide an electronic platform for researchers to manage laboratory data, encompassing text, images, graphs, and charts, among others. In this article, we will explore the advantages that ELNs offer over traditional paper notebooks.

What are Electronic Lab Notebooks used for?
Electronic Laboratory Notebooks (ELNs) provide researchers with a range of capabilities for managing their laboratory data. Some of the things you can do in an electronic laboratory notebook include:
Record experimental data
ELNs provide the ability to organize laboratory data in a logical and structured manner. Users can group data based on experiments, projects, or research topics, simplifying the process of locating and accessing specific data as needed.
Organize data
ELNs offer the capability to organize laboratory data in a logical and structured manner. Users can group data based on experiments, projects, or research topics, which facilitates the ease of finding and accessing specific data when required.
Keep a lab inventory
ELNs provide the ability to track laboratory inventory, encompassing chemicals, reagents, and equipment. Users can record the location, quantity, and expiration dates of each item, guaranteeing the availability of necessary materials for experiments.
Create protocols and procedures
ELNs enable the creation and storage of laboratory protocols and procedures. This ensures that all members of the lab adhere to the same standard operating procedures, promoting consistency and uniformity in research practices.
Generate reports
ELNs offer a range of tools for generating reports from laboratory data. Users can create graphs, charts, and tables within the ELN platform and export them to other software applications for further analysis and presentation purposes.
Overall, ELNs offer researchers a wide array of capabilities for effectively managing laboratory data. These capabilities encompass recording and organizing experimental data, collaborating with colleagues, and generating reports to streamline research processes.

Why have Electronic Lab Notebooks become critical for Research and Development in Pharma?
There are several reasons why Electronic lab notebooks (ELNs) have become indispensable in the pharmaceutical industry for research and development purposes. Some of the benefits that ELNs provide over traditional paper notebooks are:
Adept with Compliance
- ELNs provide an audit trail of all laboratory activities, ensuring compliance with regulatory requirements such as Good Documentation Practice (GDP) and Good Manufacturing Practice (GMP).
- ELNs provide a comprehensive audit trail of all laboratory activities, ensuring compliance with regulatory requirements such as GDP and GMP.
Improved Standard Practices
- ELNs enable the adoption of laboratory best practices. They facilitate the establishment and maintenance of standardized procedures for data capture, analysis, and documentation. This makes it easier to ensure consistency and adherence to protocols in the laboratory.
- By utilizing ELN, lab data becomes clear and organized, leading to improved accuracy and consistency of data entry. This, in turn, helps meet regulatory compliance requirements by ensuring that data is recorded and documented accurately and consistently.
- ELN meets compliance standards with audit trails and strict access controls. This ensures that all data is trackable and can be reviewed by authorized personnel. Simultaneously, it prevents unauthorized access to confidential information.
- ELN simplifies tracking of sample information by offering various options. Users can add files, external links, graphs, and tags to enhance the data recording process. This flexibility allows all relevant information pertaining to a sample to be easily accessible in one location.
Increased Efficiency and Reduced Error
- ELNs reduce the time and effort required for manual data entry, enabling researchers to focus on more critical tasks such as data analysis.
- ELNs facilitate the capture, organization, and analysis of laboratory data, making it easier to identify trends, generate insights, and improve decision-making.
- ELNs eliminate the risk of documentation errors that can arise from manual transcription. This reduction in errors minimizes the likelihood of compliance issues that may arise due to incorrect or missing information.

Enhanced Collaboration
- ELNs allow real-time collaboration and sharing of laboratory data, facilitating faster decision-making, and reducing the risk of miscommunication.
- ELN enhances collaboration among lab members with shared search functionality for selected scientific teams both in and out of the lab. This allows for a more efficient workflow and fosters teamwork, enabling researchers to easily share and discuss data.
Advanced Data Security
- ELNs provide a secure and centralized platform for laboratory data, reducing the risk of data loss or theft.
- ELN meets compliance standards with audit trails and strict access controls, ensuring that all data is trackable and can be reviewed by authorized personnel, while preventing unauthorized access to confidential information.
- ELN has accessibility balanced across the team and inter-institution sharing of resources, using technologies like 2-factor identification, unique passwords, IP Address restrictions, and data-protocol partitioning. This ensures that lab data is secure and accessible only to authorized personnel while facilitating the sharing of data with other institutions or departments.
Centralized data management
- Electronic laboratory notebooks (ELN) provide one centralized location for all lab data management. This ensures that information is easily accessible, updated, and organized in a secure and compliant manner, conforming to standard practices and good documentation practices.
- ELN allows for the easy consolidation of samples, reagents, lots, plates, barcodes, order numbers, and tracking information, including batch data and meta-data. This ensures that all data is easily accessible and can be searched and retrieved at any time, eliminating the risk of losing valuable data.
- ELN provides easy organization and accessibility of documents and files from anywhere with an internet connection. This eliminates the need for paper-based documentation, saves time, and simplifies record-keeping.
- ELN licensing fees often include externally hosted setup and maintenance costs, ensuring ongoing administration, upgrades, and maintenance with guaranteed reliability and document up-time. This ensures that ELN is a cost-effective solution, providing the best value for money.
Integration with other systems
- ELN integrates with complex LIMS systems, supporting both structured and unstructured data. This provides a seamless integration between ELN and LIMS, ensuring that lab data is efficiently managed and accessible.
- ELN allows the ability to export or share data in multiple formats

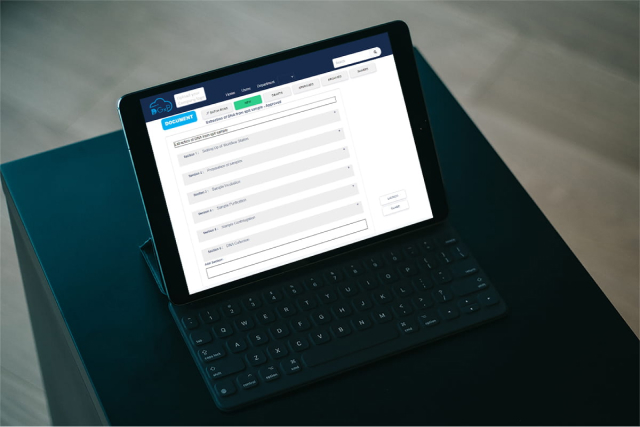
How DigitalGxP Can Help
DigitalGxP is a digital documentation software designed specifically for pharmaceutical laboratories. It is much more than a standard ELN with several additional features such as digital SOPs, video SOPs, Electronic Batch Records (EBRs), etc. With DigitalGxP, pharmaceutical companies can:
- Streamline laboratory documentation processes
- Ensure compliance with regulatory requirements
- Improve data security
- Enable real-time collaboration and knowledge sharing
- Accelerate the drug development process
So, sign up today to find out how we can assist your pharma company to streamline and digitize its laboratory documentation and data management.
In conclusion, Electronic Laboratory Notebooks are essential for research and development in the pharmaceutical industry. By adopting ELNs as standard practices in the lab, pharmaceutical companies can improve efficiency, collaboration, compliance, and data security. DigitalGxP offers a comprehensive solution for digital laboratory documentation and laboratory information management.